Received: June, 2010
Fluorine Notes, 2011, 75, 3-4
ANALYTICAL SUPPORT FOR THE SYNTHESIS OF FLUORINATED COMPOUNDS NESSESARY FOR THE PERFLUORINATED POLYMER PRODUCTION
V.G. Barabanov, N.G. Zubritskaya, V.Z. Kaufman, A.A. Trofimova
FCUE Russian Scientific Center "Applied Chemistry", 197198, Russia, St. Petersburg, Dobrolubov av. 14
e-mail: vbarabanov@rscac.spb.ru
Abstract: In this work gas chromatographic methods of the determination of perfluoro(3,6-dioxa-4-methyl-7-methyl-7-octene)sulfonylfluoride (CF2=CFOCF2CF(CF3)OCF2CF2SO2F) (monomer FC-141), raw products for it such as 2,5- di(trifluoromethyl)-3,6,-dioxa-8-sulfonylfluoride of perfluorooctanoylfluoride (FCO2CF2CF2OCF(CF3)CF2OCF(СF3)COF) (fluoroanhydride FC-161) and α-fluorosulfonylperfluoroacetylfluoride (FCO2CF2COF) (FC-41) are described. These compounds are included in the total technologyprocess of perfluorinated polymer production meant for ion exchange membrane making. The analytical method for the detection of mass concentration of the monomer FC-141, an impurity of 2-hydro-1,1,1,1,4,4,5,7,7,8,8-undekafluoro-5-trifluoromethyl-8-sulfnylfluoride-3,6-dioxaoctane (CF3CFHOCF2CF(CF3)OCF2CF2SO2F)(FC-151) and other fluorinated impurities in the monomer FC-141 at the level of 0,1 % weight has been developed. The identification of products by chromatography-mass-spectrometry and IR-spectrometry methods has been carried out.
Keywords:copolymers, β-sulton, gas-liquid chromatography
By the analytical support we mean the analysis of intermediate compounds, the identification of basic substances and impurities in them, development of analytical methods. In this work we used measuring complex consisting of two chromatographs "TSVET-800" suitable to use both with packed and capillary columns, chromatography-mass-spectrometer Shimadzu "GCMS-QP2010" with software "GCMSsolution" and mass detection range from 5 to 2000 m.e and Fourier transform infrared spectrometer Shimadzu "IR Prestige-21" .
First work in this research was the chromatographic detection of water in solvents such as diglyme, acetonitrile, tetraglyme etc. used in the synthesis of fluorinated compounds. Even the presence of water traces can essentially reduces the yield of target product and results in the appearance of additional impurities. It was required to detect the mass percentage of water at the level of 0,02 -0,04%. It is known that the gas-liquid chromatographic methods are not usually used for it. The best solution is the separation of the sample into the columns with porous polymer sorbents i.e. gas-solid chromatographic separation with a katharometer as a detector. Polisorb-1 - a product of copolymerization of styrene and divinylbenzene has been selected as a sorbent. Water is weakly kept on this sorbent and the peak corresponding to the registration of water appears directly behind the air peak as the water is (a high-polar substance) is not kept on the nonpolar surface of the sorbent because of the absence of adsorption centers on it. The analysis was carried out with temperature programming at the same time, the speed of heating was chosen for each solvent separately. Calibrating coefficients were calculated by an addition method.
First compounds in the process of the monomer FC-141 synthesis are β-sulton and its linear isomerized analog - α-fluorosulfonylperfluoroacetylfluoride (FC-41) being the parent compound for the synthesis of fluoroanhydride FC-161. Difficulty of the analysis consists in the extreme aggression of these compounds. We chose a stationary liquid phase on the basis of siloxane rubber SKTFT, high-molecular copolymer in which methyl groups in dimethylcyclosiloxane are replaced on 3-3-3-fluoropropyl groups. This phase is distinguished by the increased chemical resistance. Macroporous sorbent spherochrom S-80 is applied as a solid sorbent. The amount of applied liquid phase was 20% by weight of the sorbent. Measurements were carried out into column of 2 m in length and 3 mm in internal diameter at a column oven temperature 40°C. Oven temperature evaporator should be no more than 90 - 100 °C because at higher temperatures β-sulton could partially isomerize in the evaporator. Flow rate of carrier gas is 20 cm3/min, the detector – FID. Sample volume - 2 ml, it leads to the overloading of the substance, which in turn leads to the asymmetry of chromatographic peaks. However, to reduce the sample was impossible because of the high vapor pressure of low boiling products and the possible evaporation of the samples from the microsyringe before the injection leading to the incorrect results. It should also be considered the possibility of evaporation of the sample through the rubber gasket , as sulton traces on a needle of the microsyringe instantly broke the elasticity of gaskets
The degree of separation (K), calculated by the formula
K=Δt/(b0.5(1) + b0.5(2)) (1)
where Δt - the distance between the tops of the neighboring peaks;
bi - half-widths of
the first and second peaks.
in the case of sulton mixture separation was found to be 1.1 (Fig.
1).
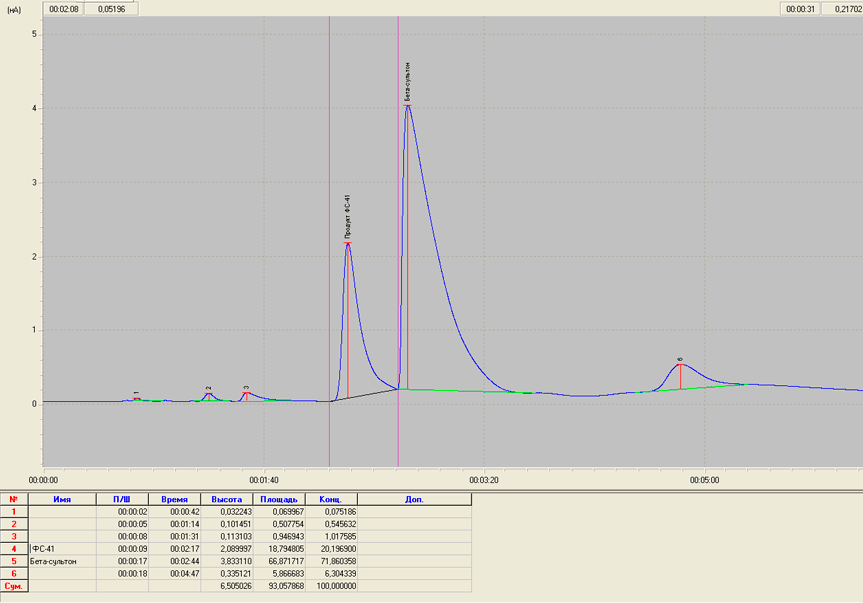
Fig.1. Chromatogram of a mixture of β-sulton and FC-41.
To perform the chromatographic analysis of the compounds of FC-161, FC-141 and FC-151 we have studied a large number of stationary liquid phases both in packed columns, and in capillary columns. The main problem was to separate the monomer of FC-141 and "hydrogen-containing" impurity FC-151 in it. The difficulty was that the FC-151 is a compound of the same class and the same structure as the FC-141, in which the group -CF=CF2 is replaced by-CFH-CF3 group, i.e. different from the FC-141 by less than 5% in molecular weight and a few degrees in the boiling point. Obviously the low-polar or non-polar liquid stationary phases are suitable in this case. As a result of our selecting efforts, it was provided that the only stationary phase ensuring the effective separation of these compounds was fluorosilicon rubber SKTFT-50. (Although it is possible that SKTFT-100 would have been better). In this case the amount of liquid phase, applied to the sorbent reached 15% by weight of the sorbent. The choice of this amount is not accidental, as we know, the quality of the column is inversely proportional to the size of sorbent particles and the thickness of the liquid phase. The size of sorbent particles used in our work is from 0.160 to 0.200 mm i.e. they are sufficiently small, and the amount of applied sorbent must be at least 10-15% since otherwise the phase would not be a regular film. This column, in length 3m, operating at a temperature of column oven 120°C and a flow rate of the carrier gas 30 cm3/min provides a degree of separation K (see formula 1) equal to 2,3.
The value of K indicates a high degree of separation of the peaks corresponding to the registration of the FC -141 and FC-151. Thus, the problem of separating of the target substance FC-141 and its main impurity FC-151 was resolved (Fig. 2).
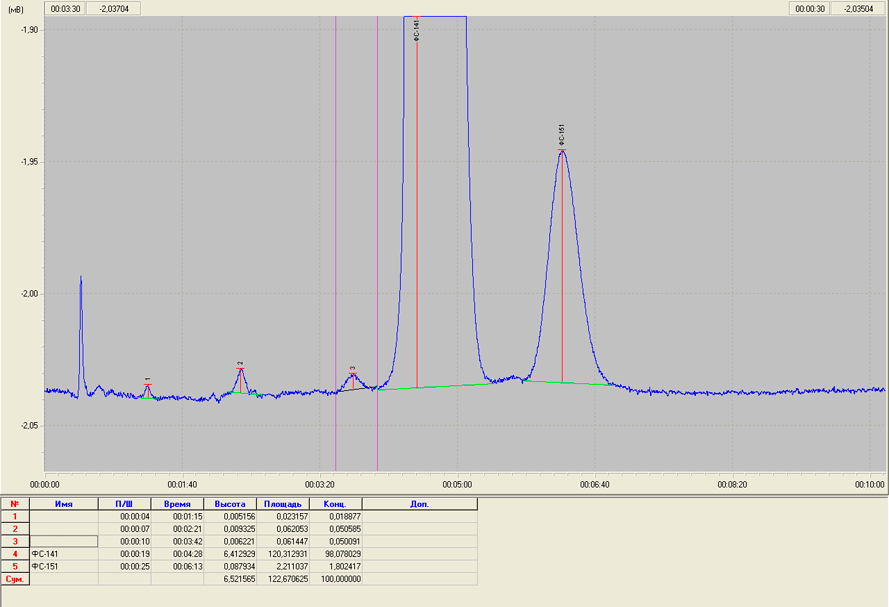
Fig.2. Chromatogram of a sample of FC-141 with its impurity FC-151.
However, due to the fact that this phase is a good solvent for the mixture components, making it possible to divide the FC -141 and FC-151, the remaining impurities release the column slowly and broad peaks. Since the requirements for the purity of FC-141 (except impurity FC-151) are extremely high (from 99.5% to 99.95%), the determination of the broad impurity peaks may have a large inaccuracy of measurements, and some of them may be omitted at all. Obviously, such requirements can not be performed on a packed column with the stationary phase SKTFT-50. We assume that this is possible either on packed columns with stationary liquid phase XE-60, or on capillary columns. Using of packed columns for these small values ??of impurity content will need to use the method of internal standard, which is inconvenient because it leads to unnecessary technical difficulties, and besides the peak corresponding to the registration of the internal standard is an large place in the chromatogram, possibly blocking peaks corresponding to small impurities. Therefore, we proposed, following the comparative experiments, to determine the impurities in the FC-141 (except the FC-151) using a capillary column with the stationary phase SE-54, thickness 20 mm, column length 35 m.
According to the results of our research the analytical method of the determination of the mass content of perfluorosulphomonomer FC-141 and impurities in it by gas chromatography was developed and certified. Determination of FC-151 was carried out using a packed column with a stationary phase SKTFT-50 by the method of internal normalization with the estimation of the calibration factor for FC-151. The other impurities were determined using a capillary column with the phase SE-54 and as these impurities had not been identified the determination was carried out by the method of absolute calibration.
With regard to fluoroanhydride FC-161, the chromatographic analysis was constantly performed in the process of the synthesis FC-141 using the same column as in determining of the FC-151 and, since the requirements for the purity of FC-161 were not so great as to FC -141 (98-99%), then all the experiments were carried out using a packed column. Chromatographic peaks corresponding to the following main compounds in the reaction mixture: dimer of hexafluoropropylene oxide, trimer of hexafluoropropylene oxide, oligomer FC-101 (FCO2CF2CF2OCF(CF3)COF), fluoroanhydride FC-161 (target compound) and oligomer FC-221 (FCO2CF2CF2OCF(CF3)CF2OCF(CF3)CF2OCF(CF3)COF). Measurements were performed at the column oven and 120°C, flow rate of the carrier gas 30 cm3/min. Relative retention times are as follows: dimer - 0.25, trimer - 0.33, FC-101 - 0.5, FC-161 - 1.0, FC-221 - 2.38.
At processing of chromatograms the method of absolute calibration was used.
Gas chromatography-mass spectrometer and infrared spectrophotometer were applied for the identification of the target and intermediate compounds. The sample injected into the chromatographer "QP-2010" divided into a capillary column SE-54 in 40 meters length at the temperature of evaporator 140°C. Mass spectrum registered chromatographic peaks was recorded at the ionizing voltage 70 eV. It is known that for the fluorinated compounds this ionizing voltage is too great and thus they collapse on a large number of fragments, often without giving a molecular peak. However, we managed to obtain a quite satisfactory mass spectra and in the case of FC-141 to register a molecular peak. A list of the most intense fragments for FC-141 is as follows: 31 - (CF+), 50 - (CF2+), 64 - (SO2+), 67 - (SOF+), 69 - (CF3+), 81 - (CF2CF+), 97 - (CF2CFO+), 100 - (CF2CF2+), 119 - (CF2CF3+), 131 - (CF2CFCF2+), 150 - (CF3CFCF2+), 349 - (C5O3SF11+), 446 - Molecular peak. (Fig. 3).
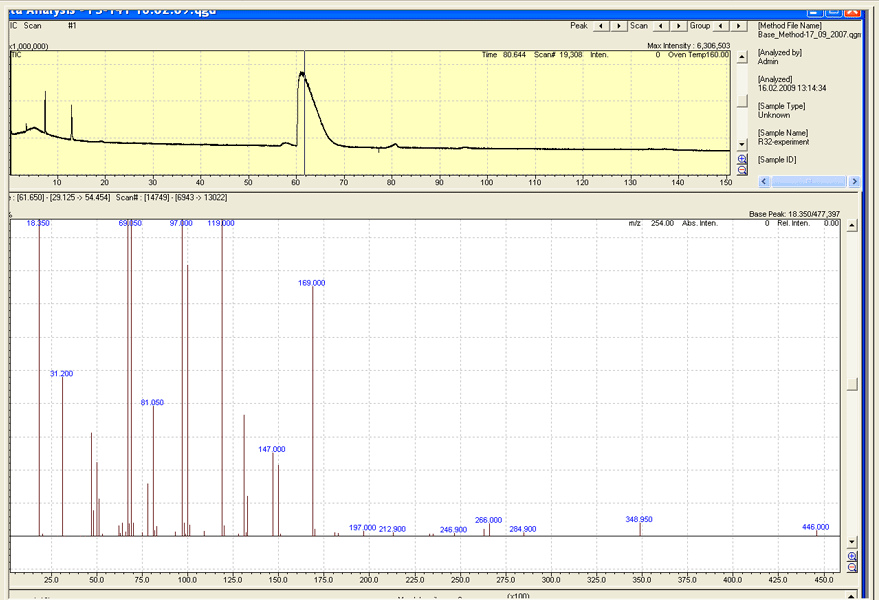
Fig. 3. Mass spectrum of a sample of FC-141.
Except the fluorinated fragments the mass spectrum of FC-151 is characterized by the essential presence of sufficiently intense masses 51 and 101, respectively, corresponding to the fragments (CF2H+) and (CF2CF2H+). The mass spectrum of fluoroanhydride FC-161 in its turn is characterized by the most heavy fragments with masses of 429 and 480, corresponding to the fragments (molecular weight - (FCO2+) and (molecular weight - (S+) respectively. Investigated compounds are fully identified by mass-spectrometry.
Investigation of the IR- spectra also provided additional information about the quality of our products. It is connected for example, with the determination of fluoroanhydride FC-161 in the monomer of FC-141. In accordance with the developed technology of production FC-161 is completely absent in FC-141. However, check it by gas chromatography we could not, because either these compounds did not separate into phases and columns available for us or the peak corresponding to FC-161 coincided with the peak of FC-151. However, the study of IR- spectra of these compounds has allowed to resolve this problem. In the IR-spectrum of FC-141 there is a rather weak absorption band corresponding to the absorption of C-O bond. Wavelength - 1838.1 cm-1. At the same time in the spectrum of FC-161 a strong band corresponding to the absorption of multiple C=O bond with a wavelength of 1884 cm-1 is registered. Prepared by us and measured calibration mixtures showed that by measuring the infrared spectra of FC-141 and comparing the above bands, you can determine the content of FC-161 in FC-141 at the level of 0.05% (Fig. 4, 5).
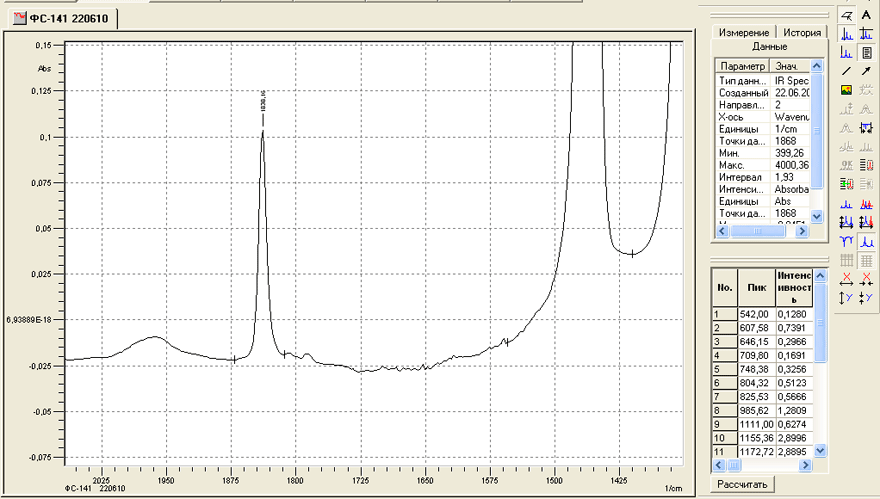
Fig. 4. IR- spectrum of a sample FC-141 in the wavelength range of 1350-2070 cm-1.
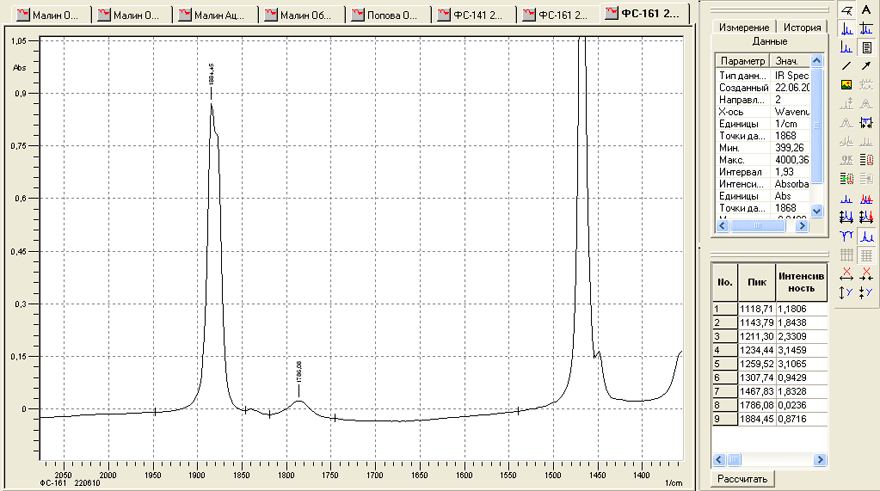
Fig. 5.IR- spectrum of a sample FC-161 in the wavelength range of 1350-2070 cm-1.
A similar situation has developed with the measurements of IR-spectra of β-sulton and FC-41. When β-sulton is isomerized in FC-41 a strong absorption band C=O appears in the IR- spectrum. It should be noted that only use of the Fourier transform infrared spectrometer has allowed to receive an informative infrared spectrum of the extremely volatile FC-41 because the total process of the spectrum registration with corresponding preparation has occupied only 15 seconds.
Thus, we can say that our available and used analytical equipment has allowed completely to solve the problem of the identification of the target compound FC-141 required to obtain the perfluorinated polymer, starting and intermediate products, impurities in them and also the determination of the mass content of the monomer FC-141.
References
- L.F. Sokolov, A.S. Odinokov, O.S. Bazanova, D.D. Moldavskiy, N.N Zatsepina, S.G. Semenov. Collection of proceedings. Anniversary issue, Fluorine compounds, Chemistry, Technology, Application. Edited by B.N. Maximov, V.I. Manuilova, V.V.Kornilov, St. Petersburg, 2009, p.109-111
- A.A. Lurie. Chromatographic materials, M., Chemistry, 1978
- Z.H. Gioshon, K. Giyemen. Quantitative gas chromatography, Wiley, 1971.
- B.V. Stolyarov, I.M. Savinov, A.G. Wittenberg. Guide to practical work on gas chromatography, Leningrad, Chemistry, 1988.
- L. Bellami. Infrared Spectra of Complex Molecules, Moscow, Foreign Literature Publishing House, 1963.
Fluorine Notes, 2011, 75, 3-4
