Received: July, 2010
Fluorine Notes, 2011, 75, 1-2
Synthesis, properties and application of F-4SF copolymer
A.S. Odinokov, O.S. Bazanova, N.G. Zubritskaya, V.V. Kornilov, B.N. Maximov and L.F. Sokolov
FCUE Russian Scientific Center "Applied Chemistry", 197198, Russia, St. Petersburg, Dobrolubov av. 14
e-mail: Bmaximov@rscac.spb.ru
Abstract: The process of radical copolymerization of tetrafluoroethylene (TFE) and perfluoro(-3,6-dioxa-4-methyl-7-octene)sulfonylfluoride (FC-141) was considered. The constants of copolymerization of TFE and FC-141 were found.
Keywords:tetrafluoroethylene, copolymer, perfluoro(-3,6-dioxa-4-methyl-7-octene)sulfonylfluoride
F-4SF is a copolymer of tetrafluoroethylene (TFE) and perfluoro(-3,6-dioxa-4-methyl-7-octene)sulfonylfluoride (FS-141) producible via in-solution radical co-polymerization [1-4], or without solvent [4,5], or by emulsion polymerization in aqua [6-13]. The co-polymerization process follows the mechanism:
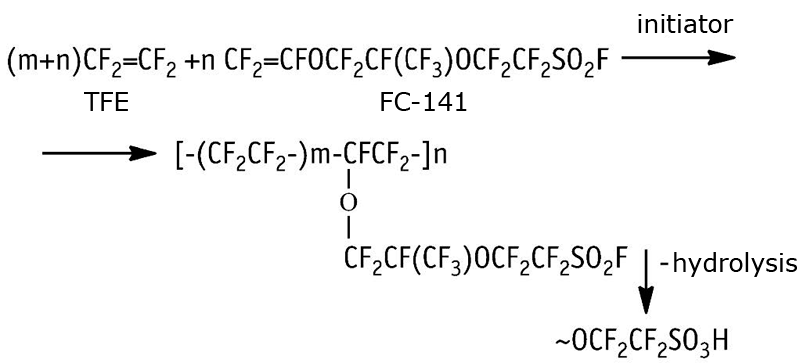
As the extrusion film is ready the copolymer sulfonylfluoride groups are converted to sulfonic acid groups through hydrolysis.
The copolymer composition is determined by its equivalent weight (EW). The EW is defined as the weight of polymer (in grams) per mole of sulfonic acid group.
The copolymer is well-known after 1960ies and applied in the proton-conductive membranes manufacture in USA (Nafion, 1962, Du Pont) and in Russia (МF-4SK, 1977) [14-16] it is also used in the production of heterogeneous acidic catalysts [17].
Each field of application specifies special requirements on the quality of F-4SF. The main properties of F-4SF governing its quality are as follows:
- number of sulfoacid groups determines both the membrane proton conductivity and the catalyst acidic activity;
- high chemical and thermal stability of the copolymer;
- average molecular weight of the copolymer determines the mechanic characteristics of items made of F-4SF.
The properties of F-4SF and items made thereof depend on the conditions of its manufacture and processing. The copolymer composition depends on the co-monomer concentration ratio in the liquid phase of the reaction mixture. FC-141 (boiling point (BP)=133.5°С) is liquid under polymerization conditions (below 100°С) and its content in the reaction mixture depends on its quantity charged into the reactor. TFE is a gas (BP=-76.5°С) and its concentration in liquid reaction mixture depends on its partial pressure in the reactor, temperature and the reaction mixture composition.
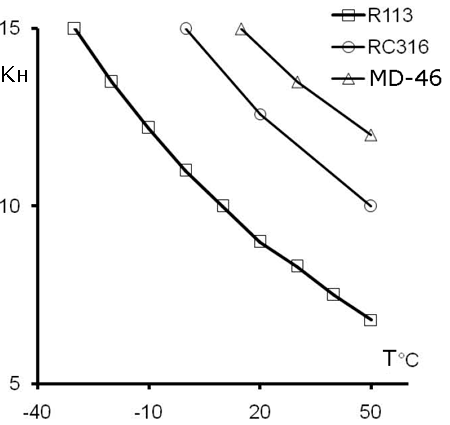
We determined TFE solubility in some fluorocarbons (1,1,2-trifluorotrichloroethane (R-113), 1,2-dichlorohexafluorocyclobutane (RC-316), perfluoro(methyldiethyl)amine (MD-46)) within working temperature range (see Fig.1, 2).
|
Fig.2. TFE solubility in R-113, RC-316(1,2-Dichlorohexafluorocyclobutane) and MD-46 (fluorinated amine). KH – Henry constant, T – temperature (°C) |
Solubility is estimated from the value of Henry constant:
KH=CL/CG,
here CL - TFE concentration in liquid phase (mole·l-1),
CG=P/22,4,
TFE concentration in gas phase (mole·l-1),
Р – TFE pressure (atm).
TFE concentration in liquid phase of the reaction mixture is calculated by the formula derived by Krichevsky [19]:
CL =KH1 *CG (mole·l-1),
KH1 - Henry constant for FС-141 mixture and solvent:
lnKH1=x1·lnKH1 + x2·lnKH2,
here x1 and x2 are parts by mole of FC-141 and
solvent in the reaction mixture, as detected by chromatography,
KH1 and KH2are Henry constant values for FC-141 and solvent at work temperature
determined from Fig.1 and Fig.2.
TFE is more soluble in fluorocarbon than in FC-141, thus making it possible to conduct solvent co-polymerization at pressure lower than that needed for FC-141 co-polymerization without solvent or for using of emulsion polymerization in aqua.
This is a benefit of the solution method for F-4SF manufacture as the process explosion risks are much less. TFE solubility grows with the fraction of fluorine in the solution (Fig. 2), and the manufacture of the same copolymer composition occurs at lower TFE pressure (Table 1), while the melt flow index (MFI) that outlines the average molecular mass of the copolymer grows as well.
Table 1. Synthesis of F-4SF in various solvents. Solvent: FS-141 = 2:1 (mass). Concentration of Bis(perfluorocyclohexanoyl)peroxide (DAP-C) initiator 6.7·10-4 mol/l. Temperature 50°С.
|
Test # |
Solvent |
Pressure, atm |
Time, hours |
TFE consumption, g |
Yield of polymer, g |
EW |
MFI, g/10min at 270°С |
|
1 |
R113 |
5,5 |
3,2 |
17 |
28 |
1000 |
21 |
|
2 |
RC316 |
4,5 |
3,3 |
17 |
30 |
1000 |
15 |
|
3 |
MD-46 |
3,5 |
3,0 |
16 |
27 |
1000 |
9 |
|
4 |
R113 |
4,5 |
4,3 |
16 |
26 |
950 |
30 |
|
5 |
RC316 |
3,5 |
5,5 |
14 |
28 |
950 |
20 |
|
6 |
MD-46 |
2,7 |
4,0 |
13 |
26 |
950 |
13 |
|
7 |
R113 |
4,0 |
5,3 |
14 |
27 |
900 |
35 |
|
8 |
RC316 |
2,5 |
5,0 |
14 |
25 |
900 |
31 |
|
9 |
MD-46 |
2,0 |
5,0 |
13,5 |
24 |
900 |
22 |
The optimal composition of F-4SF copolymer depends on its suggested application. F-4SF with EW 1200 and 1100 is suitable for the production of ion-exchange membranes to be used in chlorine-alkaline electrolysis, F-4SF with EW 950-1000 is suitable for the production of ion-exchange membranes to be used in fuel cells, and F-4SF with EW 850-950 is applied in heterogeneous acidic catalysts. Through the variation of TFE plus FS-141 co-polymerization temperature and pressure one may choose the conditions for the production of any desirable F-4SF copolymer composition (Fig.3, 4).
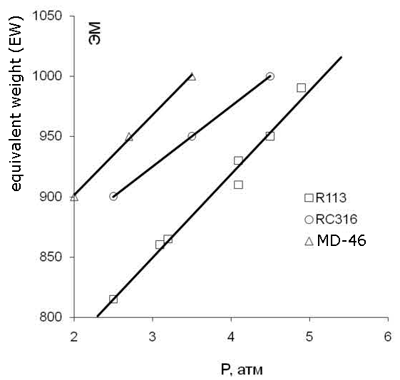
Fig.3. Dependence of F-4SF composition (EW) on the reactor pressure (Р, atm). T = 50°С.Solvent/FC-141 = 2/1 (mass). Concentration of the initiator 25·10-4 mol/l |
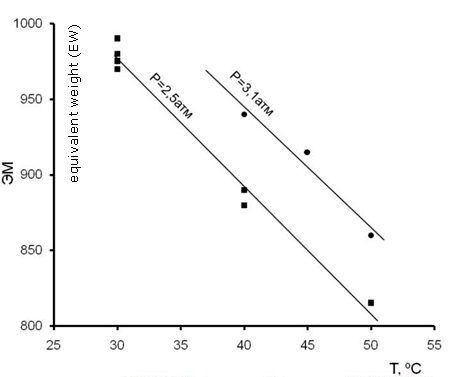
Fig.4. Dependence of F-4SF composition (EW) on the temperature of co-polymerization. R113/FS-141 = 2/1 (by mass), Concentration of initiator 30·10-4 mol/l |
We determined the constants of TFE and FC-141 co-polymerization [18]:
rTFE=9,0; rFC-141=0,04,
Those values allow the estimation of the rate of co-polymerization, average molecular weight of the co-polymer, type of the polymer chain sequencing. The values of those co-polymerization constants (rTFE>>1, rFC-141→0) testify that the co-polymer consists of TFE units interleaved with FC-141 units. The rate of co-polymerization grows exponentially with the active monomer (TFE) content in the reaction mixture [18, Fig. 4]. The co-polymer is enriched with TFE units as to compare with the monomer mixture composition [18, Fig. 3]. The rise in number of FS-141 units entails the decrease both of EW and molecular mass, while the copolymer hydrophilicity grows thus deteriorating the mechanical properties of membranes or catalysts made of F-4SF.
Thus F-4SF with EW 1000-1100 involving sulfo-acid groups (after hydrolysis) swells in water at room temperature by 10-20%. The co-polymer hydrophilicity grows with temperature, and at ~150°C it dissolves in water/alcohol, dimethylformamide, dimethylacetamide, etc. Therefore heterogenous catalyst made of F-4SF is not applicable in liquid polar media at temperature above 100°С as it swells and dissolves strongly.
The molecular mass of F-4SF produced by the water emulsion polymerization is well over that of F-4SF of the same composition but produced by the solution method. F-4SF samples produced by the solution method with EW about 1150 have MFI 30-50 at 240°С, while those produced by the emulsion polymerization have MFI 20-30 at 270°C. MFI was determined by "IIRT" device under load 2.16 kg, the jet diameter was 2.07 mm.
This is an important advantage of the water emulsion polymerization for F-4SF manufacture. However, the synthesis of F-4SF by the emulsion polymerization method results in the formation of carboxyl end-groups of the polymer, as the process is initiated by •SO-4 and •OH radicals and sulfate or alcohol groups thus formed undergo hydrolysis to carboxyl groups [20].
There is a possibility of partial hydrolysis of F-4SF co-polymer sulfonylfluoride-groups to sulfo-acid groups at the conditions of water emulsion polymerization [16].
The presence of carboxyl-, sulfate- or sulfo-acid groups in polymers increases considerably the melt temperature of those polymer sites, resulting in nonuniformity or gel formation within extrusion films, and that is a disadvantage of the water-emulsion method for F-4SF production. This flaw can be remedied if F-4SF polymer thus produced is subjected to hydrolysis, dissolving or used in the manufacture of cast membranes or supported catalysts.
Extrusion is the most efficient method for the plastic films production, usually they apply it when the viscosity of melt polymer falls in the range 103-104 Pa·s [21, p.127] and does not depend on the shear stress (Newtonian flow). The best extrusion conditions are chosen through the variation of temperature. However, for F-4SF this choice implies some difficulties. Above its melt point F-4SF reserves the residual supermolecular structure till its critical temperature Тcritical. Below critical temperature (T<Tcritical) the melt flow is not Newtonian, considerable variation in the melt viscosity occur during extrusion and it results in concentricity, variations in supermolecular structure and properties of the finish film. Extrusion of F-4SF must be conducted at temperatures above Тcritical, but when so doing the viscosity of melt co-polymer becomes to small for samples with low EW values (Fig.5 and 6).
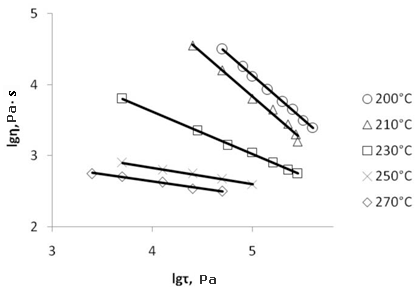
Fig.5. Dependence of the melt apparent viscosity (η, Pa·s) on the shear stress (τ, Pa) at various temperatures for F-4SF with EW 1140 in logarithmical coordinates. |
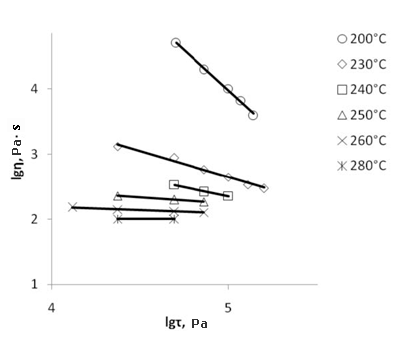
Fig.7. Dependence of the melt apparent viscosity (η, Pa·s) on the shear stress (τ, Pa) at various temperatures for F-4SF with EW 940 in logarithmical coordinates. |
Literature
- Pat. 3282875 US, US Cl 524/795. Fluorocarbon vinyl ether polymers.
- Pat. 3528954 US, US Cl 526/206. Process for homopolymerization of tetrafluoroethylene and copolymerization of same with fluoro co-monomers in the solvent 1,1,2-trichloro-1,2,2-trifluoroethane.
- Pat. 5281680 US, US Cl 526/243. Polymerization of fluorinated copolymers
- Pat. 4116888 US, US Cl 521/28. Process for producing fluorinated copolymer having ion-exchange groups.
- Pat. 4349650 US, US Cl 526/243. Polyfluoroallyloxy compounds, their preparation and copolymers therefrom.
- Pat. 62-288614 Japan
- Pat. 62-288615 Japan
- Pat. 62-288616 Japan
- Pat. 62-288617 Japan
- Pat. 4329435 US, US Cl 521/38. Novel fluorinated copolymer with tridihidrofuorosulfonul fluoride pendant groups and preparation thereof.
- Pat. 4330654 US, US Cl 526/243. Novel polumers having acid functionality.
- Pat. 4536352 US, US Cl 260/543F. Perfluoro vinyl ethers.
- Pat 2267498 Russia
- Panshin U.A., Malcevich S.G, Dunaevsksaya T.S, Fluoroplastics, Leningrad, Khimia, 1978, 230 p.
- Yamabe M, Modern Application Technology of Fluorine Compounds, Ed Ishikawa N, (Tokyo: CCMC), 1981. [11], (1981)
- Yu E Kirsh, S A Smirnov, Yu M Popkov, S F Timashev, "Perfluorinated carbon-chain copolymers with functional groups and cation exchange membranes based on them: synthesis, structure and properties", RUSS CHEM REV, 1990, 59 (6), 560–574.
- F.J. Waller, Polymer Reagents and Catalysts 308 (1986).
- Odinokov A. S., Bazanova O. S., Sokolov L. F., Barabanov V. G., Timofeev S. V..// ZhPKh. 2009. t.82. v. 1. s.113-116.
- Krichevsky V. P. //Russian Journal of Physical Chemistry, 1937, V. 9, Issue 1, p. 41-47.
- Madorskaya L. Ya., Loginova N. N., Panshin Yu. A., Lobanov A. M. Vysokomolekulyarnye soedineniya. 1983. t. A25, v.10, s. 2144-2149.
- Han Ch. D. Reologiya v processah pererabotki polimerov. M. Khimiya, 1979. 366s.
Recommended for publication by V. Kornilov
Fluorine Notes, 2011, 75, 1-2
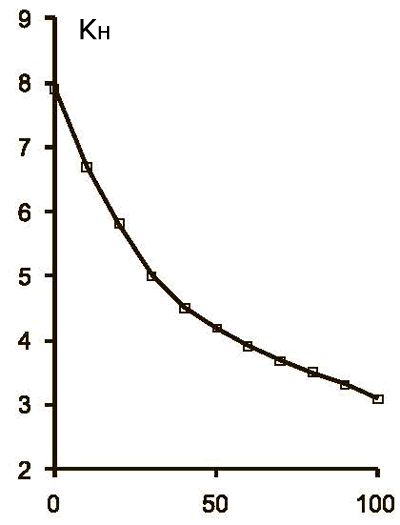 Fig.1 TFE solubility in FC-141.
KH – Henry constant, T – temperature (°C)
Fig.1 TFE solubility in FC-141.
KH – Henry constant, T – temperature (°C)